Chemicals

Over the last century humans have introduced a large number of different chemical substances into the environment. A proportion is waste from industrial and agricultural processes. Some have been designed as structural materials, whilst others are intended to perform various functions such as healing the sick or eradicating pests and weeds. Obviously some chemicals are useful yet many are toxic and their harm to the environment and our health far outweighs their benefit to society. We need to manage the risks better by only using chemicals that are safe.
Chemicals enter air as emissions and water as effluent. Industrial and motor vehicle emissions of nitrogen and sulphur oxides cause acid rain, which poisons fish and other aquatic organisms in rivers and lakes and affects the ability of soil to support plants. Carbon dioxide causes the greenhouse effect and climate change. Chlorofluorocarbons (CFCs) cause the destruction of ozone in the stratosphere and create the possibility of serious environmental damage from ultraviolet radiation. The overflow of chemical fertilisers and nutrients from farms and gardens create an accumulation of toxic algae in rivers, making them uninhabitable to aquatic organisms and unpleasant for humans. Some toxic chemicals make their way from landfill waste sites into groundwater, rivers and oceans and induce genetic changes that compromise the ability of life to reproduce and survive.
The impact of human activities on the environment is complex and affects a chain of interconnecting ecosystems. The extinction of species all along the chain may mean the loss of useful genetic material or life saving cancer drugs or safer alternatives to the dangerous chemicals in use at the moment.
Organochlorines
Organochlorine compounds such as polychlorinated biphenyls or PCB’s were originally developed for use in electric equipment as cooling agents, and are very dangerous chemicals. During the manufacture and disposal of products containing PCB’s - and as a result of accidents - millions of gallons of PCB oil have leaked out. Although their manufacture in the United States was halted in the 1970’s, and they are being phased out, they are difficult to detect, are almost indestructible and vast quantities still remain in existence. PCB contamination is extensive, and they will remain in the environment for a long time. They are detected in human milk, and also in human fat, liver and brain tissue.
PCBs accumulate in the food chain, and significant levels have been discovered in marine species - particularly mammals and sea birds - decades after their production was discontinued. Individuals who consume considerable amounts of contaminated fish, such as sports fishermen, are at particular risk of PCB exposure. Nursing infants may accumulate significant levels from human breast milk due to their small body weight. People working with old electrical equipment, or exposed to PCB fires and hazardous waste sites are more inclined to absorb PCBs via inhalation and skin contact. They are carcinogenic and capable of damaging the liver, nervous system and the reproductive system in adults. Additional toxic dioxins are formed when PCB’s are burned.
Dioxins
Dioxins are a class of super-toxic chemicals which are a by-product of the manufacture, moulding, or burning of organic chemicals and plastics containing chlorine (chlorinated organic chemicals). They are the most toxic man-made organic chemicals known, and even at levels as low as a few parts per trillion they are a major health risk. Dioxins are almost indestructible, and are only very gradually excreted by the body. They became known when Vietnam War veterans and Vietnamese civilians were exposed to dioxin-contaminated Agent Orange, and became unwell. The only other substance that is more toxic is radioactive waste.

Dioxins enter the body via food, and accumulate in body fat. They bind to cell receptors, disrupt hormone functions in the body, and they also affect gene functions. Our bodies have no defence against dioxins, and they may instigate a wide range of problems – cancer, reduced immunity, nervous system disorders, miscarriages and birth deformity. The effects can be very obvious or subtle. As they change gene functions, they may cause genetic diseases, and may also interfere with child development. Attention Deficit Disorder, diabetes, endometriosis, chronic fatigue syndrome, rare nervous and blood disorders have been linked to exposure to dioxins and PCB’s.
During the last 40 years there has been a dramatic increase in the manufacture and use of chlorinated organic chemicals. Dioxins have been discovered in high concentrations close to sites where they have been produced; and also areas where insecticides and herbicides have been heavily utilised, such as farms, orchards, or beside electric and railway lines. They have also been discovered downstream from paper mills where chlorine chemicals were used to bleach wood pulp.
In the last few years unfashionable household plastic products, together with industrial and medical waste, have been discarded by burning them in incinerators. Dioxins formed during the combustion process have been carried for hundreds of miles on tiny specks of ash, and have contaminated the countryside. They settle on pastures and crops, and are consumed by cows, pigs and chickens. They enter lakes, streams and oceans, and are taken up by fish. The food chain is also affected, and appear in meat and milk, and accumulate in the fat cells of the human body.
Cadmium
Cadmium occurs naturally in the earth's crust combined with other elements. It is generally formed as a mineral such as cadmium oxide, cadmium chloride or cadmium sulphate. Although these compounds are highly toxic they are less harmful when bound to rocks. They are present in coal and in the soil.
Cadmium is useful because it doesn't corrode easily. It is used in batteries, plastics, pigments and metal coatings. Cadmium enters the environment via landfills, inadequate waste disposal methods, and leaks at hazardous waste sites. It is produced by mining and other industrial activities. Cadmium particles enter the atmosphere when coal is burned for energy, and household waste is incinerated. These particles can travel a great distance before falling to the ground or entering water. Many tonnes of cadmium are annually discharged into our seas and oceans. Animals and plants take up cadmium when it is in the environment. If food is consumed that is contaminated with cadmium, it may irritate our digestive system and cause vomiting and diarrhoea. If inhaled it may damage the lungs. Over a period of time, cadmium accumulates in the body - even when exposure levels are low - and it is difficult to eradicate. Accumulated cadmium may cause kidney and bone disease.
We take cadmium into our body by:
Ozone
Ozone is an air pollutant. It is a colourless to blue gas with a very pungent odour.

It originates via electrical discharges from microfiche readers, laser printers and photocopiers. Electricians, electronic technicians, television and radio repairmen, and motion picture projectionists are primarily at risk from its effects. Ozone is used by the chemical industry as an oxidising agent; in the bleaching of paper, pulp, wood, flour, starch and sugar; processing certain perfumes; drying printing inks; deodorising of feathers; in the treatment of industrial wastes and as a food disinfectant.
Ozone is also created by the action of sunlight on the oxides of nitrogen, hydrocarbons or volatile organic compounds. The oxides of nitrogen are released in the emissions of vehicle exhausts and the domestic and industrial burning of gas, oil and coal. Hydrocarbons are released as vapours from petrol, industrial and household products containing solvents - for example paints.
High levels of ozone exposure may result in respiratory distress, difficulty in breathing, cyanosis (bluish skin due to a lack of haemoglobin in the blood), pulmonary oedema (swelling in the lung). Low levels may cause shortness of breath, irritation to the eyes and mucous membrane, dry nose and throat, fatigue, headache, dizziness, confusion, nausea and an unproductive cough. It leaves an acrid taste and smell. Repeated ozone exposure may result in respiratory disease.
It exacerbates breathing problems such as asthma or rhinitis, particularly in children and the elderly. The toxicity of ozone is increased by its interaction with other environmental oxidants. It also damages crops and trees.
Conclusion
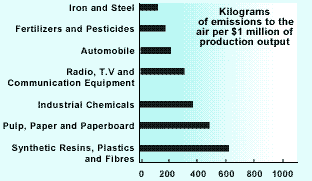
Potentially dangerous chemicals such as these are being continually introduced into the environment. As in the case of PCBs their effect on living things may not be known until many years after their release. Hundreds of thousands of different chemicals are marketed worldwide. Of those, 5000 are produced in quantities over 10 tonnes per year, and 1500 are produced in quantities over 1000 tonnes year.
There is not enough information about the environmental effects of these industrial chemicals, and their effects on humans. The balance between human activity and ecological sustainability is wrong.
What you can do
Use biodegradable products. Make your own cleaning agent using safe materials. Dispose of chemical waste carefully, and do not put them down the sink. Be wise with home maintenance and in the garden. Do not burn plastics.
Avoid organic chemicals that have ‘chloro’ as part of their names, including wood preservatives, herbicides and insecticides. Also avoid chlorine bleach (sodium hypochlorite) and products containing it. Instead, use oxygen bleach. Use unbleached paper products. Avoid ‘Permethrin’ flea sprays for pets. Avoid products made of or packaged in polyvinyl chloride (PVC), and also avoid cling film plastic wraps, unless they are clearly identified as non-chlorinated plastic.

To minimise the risk of dioxins accumulating in the body, avoid all full-fat dairy products and fatty meats such as beef or pork. Wash all fruit and vegetables to remove chlorophenol pesticide residue. Avoid grapes and raisins, unless they are clearly labelled as organically grown. Avoid soaps, toothpaste and deodorants containing ‘triclosan’, a chlorophenol.
We can reduce the dioxins in the environment if we stop producing PVC’s and other chlorinated chemicals. If your local government sends its waste to an incinerator, write a letter or email requesting that they halt the burning of plastics, and to introduce a comprehensive recycling service. Write a letter or email to companies and request them to use safe substitutes to chlorinated plastics. Ask your supermarket to sell Totally Chlorine Free (TCF) products. Join or form a local Community environmental group campaigning against hazardous chemicals.
Write a letter or email to the editor of your local newspaper. Urge him or her to publish your concerns about local chemical use and recycling issues.
Those who work with cadmium should take care not to inhale the dust, and they should avoid carrying it home from work on their clothes, skin or hair. It is also recommended to consume a wider range of foods to prevent the risk of ingesting toxic levels of cadmium.
Links:
Search our database for the contact details of organizations that directly address Chemicals






